Tip 1: How to get chloroethane from ethanol
Tip 1: How to get chloroethane from ethanol
The chemical formula of ethyl alcohol (ethanol) -C2H5OH. A substance chloroethane, used as a coolant and for anesthesia for medical purposes, has the formula C2H5Cl. These substances are similar in composition, only in the first case, the hydroxyl group is attached to the ethyl radical C2H5, and in the second - the chloride ion. You can chemically get both ethanol from chloroethane, and chloroethane from ethanol.

Instructions
1
There are a number of ways to produce chemicalconversion of chloroethane from ethyl alcohol. For example, it is possible to subject a container of ethanol to strong heating in the presence of concentrated sulfuric acid. Then, the resulting ethylene gas is combined with gaseous hydrogen chloride. Here is the scheme of the first stage of the reaction: C2H5OH = C2H4 + H2O.
2
The resulting water is absorbed by the concentratedsulfuric acid, which is very hygroscopic. Gaseous ethylene is collected in another vessel connected to the reaction flask using a glass adapter.
3
When the resulting ethylene reacts with gaseous hydrogen chloride, chloroethane forms. Here is the scheme of the second stage of the reaction: C2H4 + HC1 = C2H5Cl.
4
This reaction takes place in the presence of a catalyst- iron trichloride. By the way, in industry, chloroethane is produced in this way (of course, without using ethyl alcohol as a raw material).
5
It is possible to obtain chloroethane using a reactioninteraction of ethyl alcohol with phosphorus pentachloride. After mixing these substances, the reaction mixture is poured into water, and by means of a separatory funnel, the organic part (chloroethane) separates from the inorganic part due to the fact that chloroethane mixes very poorly with water. The reaction proceeds as follows: C2H5OH + PCl5 = C2H5Cl + HCl + POCl3.
6
There is another popular laboratory methodthe production of chloroethane from ethyl alcohol. When ethanol is reacted with thionyl chloride, chloroethane, hydrochloric acid and gaseous sulfur dioxide are obtained. Separate the organic phase from inorganic can, as in the previous example, using a separatory funnel. The reaction proceeds according to the following scheme: C2H5OH + SOCl2 = C2H5Cl + HCl + SO2.
Tip 2: How to get ethylene
Ethylene Is a combustible gas, it has a weak odor. Ethylene used in the production of hydrolysis ethylalcohol, ethylene glycol (the main part of antifreeze), styrene, polyethylene and much more. It is obtained by pyrolysis (heating without access to air) of petroleum fractions, for example, straight-run gasoline and the like. But there are ways to produce ethylene without the use of petroleum products.

You will need
- Ethyl alcohol, aluminum oxide, sulfuric acid, laboratory glassware.
Instructions
1
In a container of heat-resistant material, placea little alumina and cover it with a lid with two gas vent tubes, one of which is placed in a tube with concentrated sulfuric acid. Heat the container on the gas burner, the temperature of the alumina should be about 350 to 500 degrees.
2
Next, in a separate tube, pour a littlepure ethyl alcohol. Close the tube with a stopper with a gas outlet tube and heat it on an alcohol burner. Disconnect the gas pipe with the container in which the aluminum oxide is located. When heated, alcohol will evaporate, passing through the gas outlet, enter the vessel with aluminum oxide, and at a high temperature on aluminum oxide, dehydration will occur, i. E. splitting of water from molecules of alcohol. Ethylene with water vapor and unreacted alcohol in the gaseous state will leave the vessel. This mixture will fall into a test tube with sulfuric acid, which serves to dehydrate the mixture.
3
Mix ethyl alcohol and concentratedsulfuric acid. A reaction will occur to form the acid ethyl ester. Heat the mixture, when heated, there will be a process of dehydration of alcohol with evolution of ethylene.
Tip 3: How to separate alcohol from water
Alcohol, used in everyday life, is known as ethanol. However, the liquid that results from the process of alcohol fermentation contains ethanol and water. To separate alcohol from water you can use the process of distillation.

You will need
- - distillation flask;
- - stand;
- - Bunsen burner;
- - thermometer;
- - fractional column;
- - the Liebig capacitor;
- - potassium carbonate;
- - conical flask;
- - gas source with a tap;
- - rubber hose;
- - matches
Instructions
1
Pour in a mixture of alcohol and water in a distillation flask, which, as a rule, is glass with a round bottom. Place the flask on the stand over the Bunsen burner. To produce an open flame in a Bunsen burner, gas is used.
2
Attach the straight end of the fractionator tothe opening of the distillation flask in such a way that the thinner curved end of the fractionation column is directed downward. Insert the thermometer into the top of the column. It is necessary to control the process temperature, which should not exceed 73.3 degrees Celsius.
3
Connect the Liebig capacitor to the thin, curved end of the fractional column. The condenser will cool the vapor to the temperature of its transition point to the liquid state.
4
Place the potassium carbonate in the open outlet of the Liebig condenser. Potassium carbonate will absorb all water passing through the condenser.
5
Place the conical flask under the outlet of the Liebig condenser. In this flask will be collected alcohol, separated from the original mixture of liquids.
6
Connect the burner to the gas source, securingone end of the rubber tube with screws to the gas supply tap, and the other to the Bunsen burner. Turn the ring at the bottom of the burner to the "On" position. Turn on the gas source by turning the nozzle so that it is parallel to the rubber tube.
7
Light a match and set fire to the gas. Adjust the intensity and height of the flame by turning the ring on the burner until a blue flame is obtained. The flame will heat the mixture of alcohol and water, starting the distillation process.
8
The liquid that accumulates in the conical flask will be a 95% solution of ethanol (or alcohol). Continue the process until the liquid separates from the condenser.
Tip 4: How to get ethylene from ethanol
Ethanol, or ethyl alcohol, like ethylene refer to organic compounds. Ethanol is a monohydric alcohol, and ethylene Is an unsaturated hydrocarbon of the alkene class. However, there is a genetic link between them, according to which one can be obtained from one substance, in particular from ethanol - ethylene.

You will need
- - device for the production of ethylene;
- - concentrated sulfuric acid;
- - ethanol;
- - bromine water or potassium permanganate;
- - heating device.
Instructions
1
Ethyl alcohol is a colorless liquid with a characteristic smell of alcohol. It is ethanol used to produce ethylenea. This experience is considered affordable and safe enough even for the school chemistry course. Ethylene is a gaseous substance that is not visually detectable. However, its presence proves qualitative reactions to unsaturated hydrocarbons.
2
To carry out the experiment, take a tube with a stopper and a gas outlet tube. Secure the device to receive ethylenebut in a laboratory tripod. Pour 2-3 ml of ethyl alcohol into the test tube. Very carefully add there the concentrated sulfuric acid, which should be taken in an amount 2 times the volume of alcohol (that is, 6-9 ml).
3
Since it will be necessary to heat, inThe resulting mixture must be added a little clean (pre-calcined and free of impurities) sand. It will protect the mixture from ejection from the container. Plug the tube and begin to heat it. Concentrated sulfuric acid has a water-removing property that allows it to "take" water. As a result, a dehydration reaction occurs, i.e., water splitting. As a result, a gaseous substance is formed - ethylene.
4
Since it can not be seen, then to confirm the reaction, experience. To do this, skip the flow ethylenebut through bromine water having a brown color. There will be a discoloration of bromine water, which indicates that a halogenation reaction (in particular, bromination) ethylenea. This reaction is qualitative for unsaturated hydrocarbons, namely, ethylene.
5
Since bromine water is a very toxic compound,then it can be replaced with potassium permanganate (ordinary potassium permanganate). Prepare a dilute solution of potassium permanganate, acidify it with sulfuric acid and pass through it ethylene. There will be discoloration of the solution, which also indicates the presence of ethylenea, which was formed in the first experiment.
Tip 5: What is the chemical formula for ethanol
Ethyl alcohol is a clear, colorless liquid with a characteristic odor. Its density is about 0.8 grams per cubic centimeter. And what is the chemical formula of ethyl alcohol?
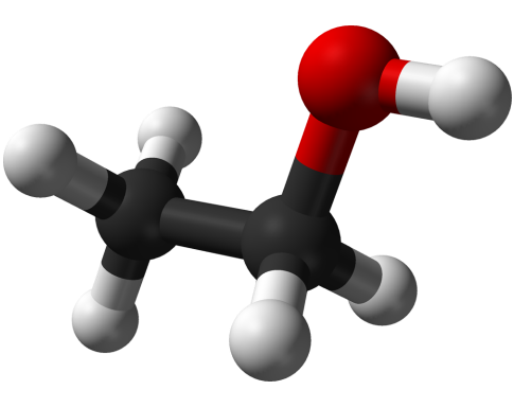
Ethanol ("ethanol" according to the internationalchemical classification) is widely used in medicine, as a disinfectant, as well as in some areas of industry as a solvent, fuel, a component of antifreeze. In addition, ethanol is the main active ingredient of alcoholic beverages.
Why is the structural formula of ethanol insufficiently accurate
The formula of any chemical shouldContain information about how many and what atoms are contained in its molecule. Ethyl alcohol consists of three elements: carbon (C), hydrogen (H) and oxygen (O). Each ethanol molecule includes 2 carbon atoms, 6 hydrogen atoms and 1 oxygen atom. Consequently, the empirical (simplest) formula of this chemical compound is written in this way: C2H6O. It would seem that this is quite enough. However, the use of an empirical formula alone can lead to an error. The fact is that exactly the same formula C2H6O applies to another substance - dimethyl ether, which is under normal conditions in a gaseous state, and not in a liquid like ethanol. And, of course, the chemical properties of this substance also differ from the properties of ethyl alcohol.Therefore, it is impossible to use just one empirical formula for the description of ethyl alcohol.
What is the structural formula of ethanol
In such cases, more accurateStructural formulas that contain information not only about the number and type of atoms of elements in a molecule, but also about their location, mutual relations. The structural formula of ethanol is: C2H5OH or even more precisely - CH3-CH2-OH. This formula indicates that the ethanol molecule consists of two main parts: the ethyl radical C2H5 and the hydroxyl radical (it is often called the hydroxyl group) OH. With the help of the structural formula, one can draw a conclusion about the chemical properties of a substance due to the presence in its composition of a very active hydroxyl group, toward which, due to the oxygen atom, the second electron electronegativity (after the fluorine) element, the electron density of the molecule is shifted.For comparison, the structural formula of the dimethyl ester CH3-O-CH3 mentioned above. That is, it is a symmetrical molecule.The formula C2H5OH is very simple and is usually remembered very easily, it reads "Tse two ash five about ash."







