How to make a galvanic cell
How to make a galvanic cell
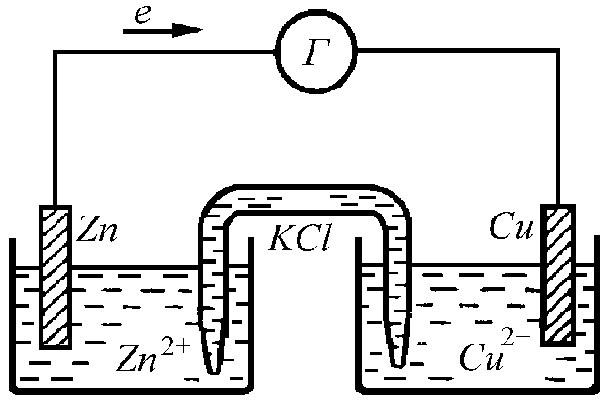
Galvanic element called a chemical current source. From several such elements it is possible to make a battery, which gives the necessary voltage for powering the appliance. The simplest galvanic element is called element Callo. You can do it at home or in a school laboratory.

You will need
- - glass or can of glass;
- - copper wire or lead plate;
- - strip or rod of zinc;
- - water;
- - salt;
- - a little sulfuric acid;
- - copper vitriol;
- - a voltmeter or a tester;
- - measuring chemical dishes;
- - scales;
- - Locksmith tools.
Instructions
1
Strip the copper wire to a shine. This can be done with a file or sandpaper. Twist from it an arbitrary concentric spiral with a diameter slightly less than the diameter of the bottom of the glass. Put the spiral on the bottom, and put the free end above the edge of the glass.
2
From zinc, cut out a strip, the length of which is 1-2cm is more than the height of the glass. Bend it so that it can hang on the edge of the glass, and the other end, located inside the vessel, would not reach to the bottom and the copper electrode lying on it by about 1 cm.
3
4
Pour a small amount into the glasscrystalline copper sulfate so that it forms a thin layer (1-2 mm) on a copper electrode. The solution should be a transparent liquid. The upper part of it remains colorless, and the lower part is painted in a dark blue color.
5
Take the measuring instrument (laboratoryvoltmeter, tester, meter and multimeter) and connect it with the help of "crocodile" clips to the electrodes of the electrochemical cell. In this case, connect the zinc electrode to the terminal marked "-", and the copper electrode, respectively, to "+". The Kallo element develops an EMF equal to 1 V, and has an internal resistance of about 2-3 Ohms. Such elements can be assembled into a battery that provides enough voltage to power a transistor receiver, a small flashlight and other small devices.